Monday : The pharmaceutical firm Regeneron announced it entered in the stage of its human clinical trials investigating a drug to both treat and prevent COVID-19.
The drug, called REGN-COV2,and this drug is a combination of two antibodies that block the coronavirus’ “spike protein” which it uses to invade human cells.
Read Also – Approx three digit Japanese companies affected by U.S. work visa halt
The company is moving to the final Phase 3 stage of a trial to determine if its drug can prevent infection among people recently exposed to the virus — for example through a person in their household.
This trial is a joint effort of the US National Institute of Allergy and Infectious Diseases (NIAID) , and is expected to enroll around 2,000 patients in the U.S
Regeneron President George Yancopoulos said, “We are pleased to collaborate with NIAID to study REGN-COV2 in our quest to further prevent the spread of the virus with an antiviral antibody cocktail that could be available much sooner than a vaccine.
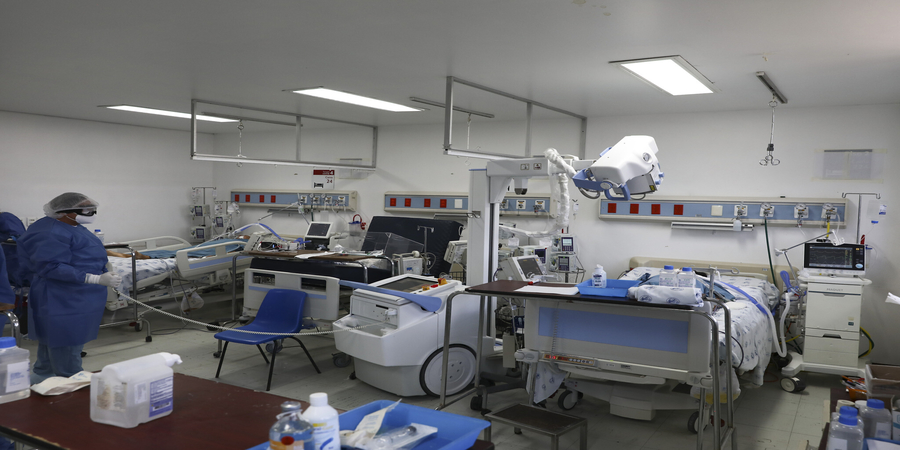
At the same time, Regeneron announced it was moving to the final stages of a trial to determine the drug cocktail’s ability to treat both hospitalized and non-hospitalized COVID-19 patients.
With preliminary data expected later this summer, This will involve around 1,850 hospitalized and 1,050 non-hospitalized patients in the US, Brazil, Mexico and Chile.
Last year, a triple antibody cocktail developed by Regeneron was shown to be effective against the Ebola virus.

![[商品価格に関しましては、リンクが作成された時点と現時点で情報が変更されている場合がございます。] [商品価格に関しましては、リンクが作成された時点と現時点で情報が変更されている場合がございます。]](https://hbb.afl.rakuten.co.jp/hgb/1c2c0a0d.31741f18.1c2c0a0e.fdc967de/?me_id=1332214&item_id=10000051&pc=https%3A%2F%2Fthumbnail.image.rakuten.co.jp%2F%400_mall%2Fkaei-trading%2Fcabinet%2F05248292%2F07086809%2Fimgrc0091198595.jpg%3F_ex%3D240x240&s=240x240&t=picttext)


